Journal:
Cancer Research (February 18, 2021);
DOI: 10.1158/0008-5472.CAN-20-264
Authors:
Abstract
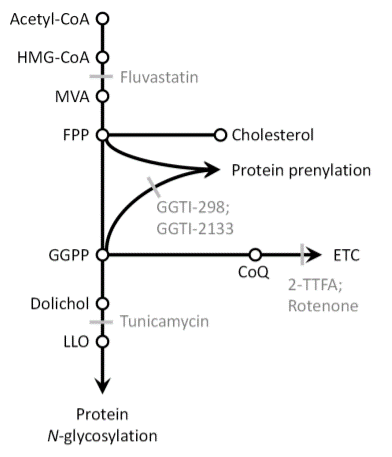
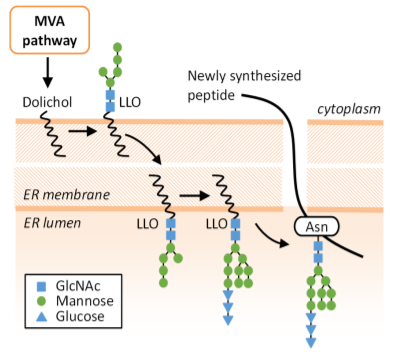
Aberrant N-glycan Golgi remodeling and metabolism are associated with epithelial-mesenchymal transition (EMT) and metastasis in breast cancer patients. Despite this association, the N-glycosylation pathway has not been successfully targeted in cancer. Here we show that inhibition of the mevalonate pathway with fluvastatin, a clinically approved drug, reduces both N-glycosylation and N-glycan-branching, essential components of the EMT program and tumor metastasis. This indicates novel crosstalk between N-glycosylation at the endoplasmic reticulum (ER) and N-glycan remodeling at the Golgi. Consistent with this cooperative model between the two spatially separated levels of protein N-glycosylation, fluvastatin-induced tumor cell death was enhanced by loss of Golgi-associated N-acetylglucosaminyltransferases MGAT1 or MGAT5. In a mouse model of post-surgical metastatic breast cancer, adjuvant fluvastatin treatment reduced metastatic burden and improved overall survival. Collectively, these data support the immediate repurposing of fluvastatin as an adjuvant therapeutic to combat metastatic recurrence in breast cancer by targeting protein N-glycosylation at both the ER and Golgi.