Journal:
Nature Communications. 2018 August; 9(1): p. 3502
Authors:
Dharmendra Dingar, William B. Tu, Diana Resetca, Corey Lourenco, Aaliya Tamachi, Jason de Melo, Kathleen E. Houlahan, Manpreet Kalkat, Pak-Kei Chan, Paul C. Boutros, Brian Raught and Linda Z. Penn.
Abstract:
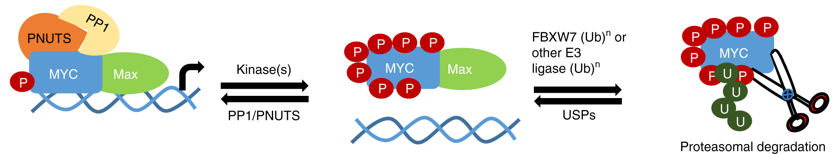
The c-MYC (MYC) oncoprotein is deregulated in over 50% of cancers, yet regulatory mechanisms controlling MYC remain unclear. To this end, we interrogated the MYC interactome using BioID mass spectrometry (MS) and identified PP1 (protein phosphatase 1) and its regulatory subunit PNUTS (protein phosphatase-1 nuclear-targeting subunit) as MYC interactors. We demonstrate that endogenous MYC and PNUTS interact across multiple cell types and that they co-occupy MYC target gene promoters. Inhibiting PP1 by RNAi or pharmacological inhibition results in MYC hyperphosphorylation at multiple serine and threonine residues, leading to a decrease in MYC protein levels due to proteasomal degradation through the canonical SCFFBXW7 pathway. MYC hyperphosphorylation can be rescued specifically with exogenous PP1, but not other phosphatases. Hyperphosphorylated MYC retained interaction with its transcriptional partner MAX, but binding to chromatin is significantly compromised. Our work demonstrates that PP1/PNUTS stabilizes chromatin-bound MYC in proliferating cells.
Link: https://www.nature.com/articles/s41467-018-05660-0
DOI: 10.1038/s41467-018-05660-0